
연구
Research Outcome
미래를 창조하는 포스텍 화학공학과
Anion Effects on Crystal Water Reactivity and Cathode-Electrolyte Interphase of Prussian Blue in Sodium-Ion Batteries
- Title of paper
- Anion Effects on Crystal Water Reactivity and Cathode-Electrolyte Interphase of Prussian Blue in Sodium-Ion Batteries
- Author
- [조창신 교수님 연구실] 나트륨이온 배터리용 프러시안 블루의 결정수 반응성과 계면 특성에 미치는 음이온의 영향
- Publication in journal
- Small methods
- Publication date
- 20250625
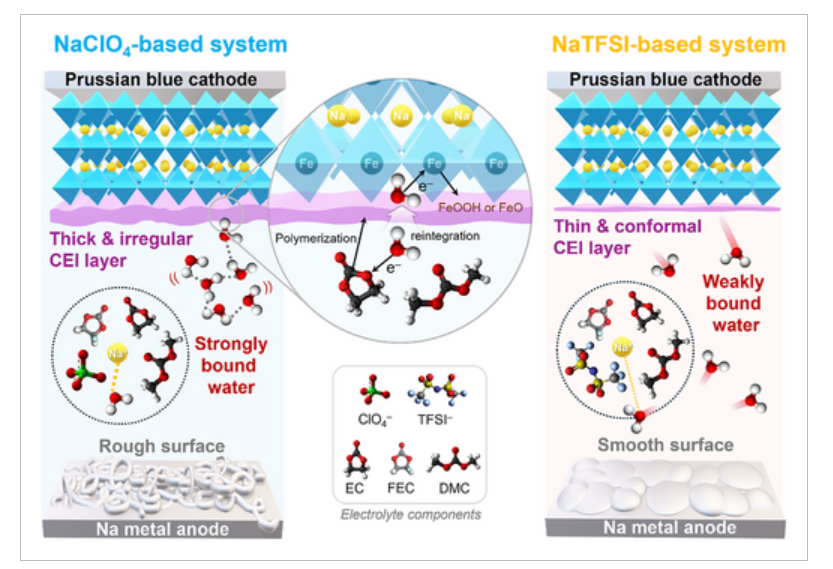
Abstract
Prussian blue (PB) is a promising low-cost cathode material for sodium-ion batteries (SIBs), but the impact of crystal water on performance degradation remains unclear. This study explores how PB's crystal water interacts with different electrolyte salts—NaClO4 and NaTFSI—affecting solvation structure and interfacial stability. Based on the Hofmeister series, it is demonstrated that the strong hydration of ClO4– sustains water reactivity, promoting Fe oxidation and solvent decomposition at high voltages. In contrast, the weakly hydrated TFSI– suppresses water-induced side reactions and facilitates the formation of stable interphases on both cathode and anode. Electrochemical analysis at 4.0 V and 4.2 V revealed that NaTFSI consistently improves reversibility, particularly at 4.2 V, achieving 77.1% capacity retention over 500 cycles—56.8% for NaClO4. The results highlight the crucial role of electrolyte-dependent water coordination in determining PB electrode stability, offering valuable insights for designing electrolytes and interphases for long-life PB-based SIBs.
DOI: https://doi.org/10.1002/smtd.202500827
Link: https://onlinelibrary.wiley.com/doi/10.1002/smtd.202500827



