연구
Research Outcome
미래를 창조하는 포스텍 화학공학과
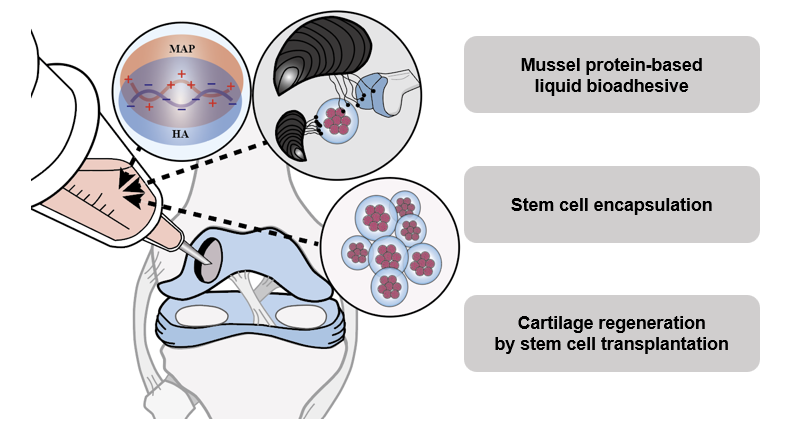
Adipose stem cell transplantation using adhesive protein-based viscous immiscible liquid for cartilage reconstruction
- Title of paper
- Adipose stem cell transplantation using adhesive protein-based viscous immiscible liquid for cartilage reconstruction
- Author
- [차형준 교수 연구실] 연골 재생을 위한 접착단백질 기반의 액상접착제를 이용한 줄기세포 이식기술 개발
- Publication in journal
- Chemical Engineering Journal, 463, p142379 (2023)
- Publication date
- 20230301
Abstract
Articular cartilage (AC) is the smooth tissue that covers the end of bones and absorbs external impacts. AC can be damaged by various types of impacts. Thus, damaged AC can trigger the development of osteoarthritis (OA). With the limited intrinsic healing capacity of AC, cell transplantation has been recognized as a possible means of AC reconstitution. However, transplanted stem cells disappear from the transplantation site rapidly, abrogating the original intent of cell transplantation. In the present work, a viscous immiscible liquid-phase bioadhesive using bioengineered mussel adhesion protein (MAP) was applied for prolonged retention of transplanted stem cells in defective AC and retained stem cells in situ. Through electrostatic interactions with high-molecular-weight hyaluronic acid (HA), MAP was formulated into a viscous immiscible liquid phase complex coacervate (MAP-HA) with self-encapsulation of stem cells. MAP-HA exhibited excellent rheological and adhesive properties without cytotoxicity. Through in vivo rabbit OA model evaluation, we proved that immobilizing transplanted cells and preventing dispersion into the joint cavity by MAP-HA can increase the survival of transplanted cells and enhance their therapeutic effects. Therefore, stem cell-encapsulated biocompatible MAP-HA can be used as a cell-retaining bioadhesive scaffold in effective cartilage regeneration.